Blog Post 10 – Yellow Discoloration of a Drug Product – How a Database Similarity Search Reveals the Culprit
3 min reading time
A customer contacted us for analytical assistance to find the substance responsible for yellow discoloration of a drug stored in polyethylene (PE) bottles. The coloration increased over time during storage in the PE bottle, while there was no coloration of the drug that was stored in inert glass containers. This observation already indicates that a leachable slowly migrating from the packaging may cause the discoloration. Not meeting a color specification for the drug product immediately results in a failure for batch release. Hence a root cause analysis was initiated. In order to find the compound or compounds that may be responsible for the discoloration, non-target analysis was performed both using Gas Chromatography-Mass Spectrometry (GC/MS) and Liquid Chromatography-Mass Spectrometry (LC/MS) screening methods to detect a wide range of organic compounds. Figure 1 shows the GC/MS chromatogram of the yellow test sample (in PE bottle) and the clear control sample (in glass bottle).
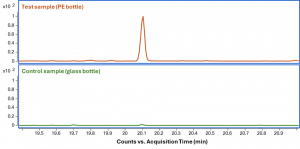
Figure 1: GC/MS chromatogram of the test sample (top, orange) and the control sample (bottom, green) on the same intensity scale.
When comparing both chromatograms, a significant peak was detected at 20.1 min in the test sample which was much less abundant in the control sample. The corresponding mass spectrum was searched against our in-house GC/MS screening database as well as the NIST23 database. No hits were obtained from this identity search, meaning that the compound was not present in the searched databases.
However, the NIST MS search program also allows to perform a similarity search to find compounds in a spectral library that are structurally similar to an unknown compound. Figure 2 shows the result of a similarity search of the unknown MS spectrum (in red) with the NIST23 reference spectrum of 3,5-di-tert-butyl-4-hydroxybenzyl alcohol.
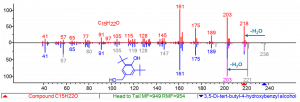
Figure 2: MS spectrum of the peak at 20.1 min in the test sample (top, red) and the NIST23 reference MS spectrum of 3,5-di-tert-butyl-4-hydroxybenzyl alcohol (bottom, blue).
Upon examination of both mass spectra, they are nearly identical from the lowest masses up to m/z 189. The difference is in the molecular ion (M) and methyl loss from the molecular ion (M-15). For the unknown MS spectrum this is m/z 218 and 203 and for the reference spectrum this is m/z 236 and 221, respectively. The mass difference corresponds to 18 Da which can be most probably explained by the loss of 1 water molecule (H2O, 18 Da). This is visualized by the NIST MS search program where the original peaks of m/z 236 and 221 are greyed out and shifted -18 Da to predict a new spectrum (pink lines) for a compound that differs -18 Da with the reference compound. This way, a hybrid match is obtained with the unknown MS spectrum.

Figure 3: Reaction of 3,5-di-tert-butyl-4-hydroxybenzyl alcohol (left) to the proposed structure for the unknown compound, BHT quinone methide (right).
A structure proposal for the unknown compound was inferred by loss of a water molecule from 3,5-di-tert-butyl-4-hydroxybenzyl alcohol, where the resulting structure was proposed to be BHT quinone methide (ref. 1). It is reported in literature that BHT quinone methide itself is dark yellow to brown and may be responsible for yellowing of polymers and solutions (ref. 2).
BHT quinone methide is a well-known oxidation product of BHT, used as antioxidant in many polymer formulations. From these results, Nelson Labs recommended to the customer to evaluate the leaching behavior of both BHT and BHT quinone methide from the bottle. Additionally, the potential oxidative pathways (light, thermal or chemical) that may cause the formation of BHT quinone methide should be reviewed. Lastly, BHT quinone methide itself is a reactive chemical, easily reacting with nucleophilic groups that may be present on the drug substance or an excipient. Hence, one should be careful with BHT as antioxidant used directly in drug products or as leachable from the primary packaging (ref. 3).
References:
[1] Chasar, Dwight W.; Westfahl, J.C., Journal of Organic Chemistry 42 (1977), 2177-2179.
[2] Pospı́šil, J. et al., Polymer Degradation and Stability 78 (2002), 251-255.
[3] Willcockson, M.G. et al., Journal of Pharmaceutical Sciences 102 (2013), 3579-3585.
If you have additional questions about Impurities Identification test services or would like to consult with the experts at Nelson Labs, just send an e-mail to [email protected].



