Blog Post 4 – When one dimension isn’t enough: unraveling impurities from secondary packaging
2 min reading time
In our ongoing series on impurities in drug products, we encountered a compelling case that highlights the hidden risks of packaging changes—even when the packaging isn’t in direct contact with the drug product.
A secondary packaging component was replaced, and it still led to the appearance of an unexpected impurity. As already shown in our first case study on impurities from a label, this phenomenon isn’t rare: chemical compounds can migrate through primary packaging and end up in the drug product, posing a challenge for Quality Control (QC).
The routine QC method in place used mobile phase modifiers incompatible with Mass Spectrometry (MS), limiting our ability to identify the impurity directly. At this point, we had two options:
- Translate the existing method to an MS-compatible version, attempting to preserve retention behavior, for instance by replacing the mobile phase modifiers.
- Use Heartcut-2DLC-MS. This technique allows to analyze the sample with the original QC method, then isolate the unknown UV peak of interest and inject it onto a second-dimension Liquid Chromatography (LC) column with MS-compatible mobile phases. Coupling the latter to a high-end MS detector enables mass spectrometric identification of the impurity.
The first strategy is relatively straightforward in execution and may be the preferred choice in many cases (see also our previous blog post). However, mobile phase modifiers play a critical role in the retention behavior of both Active Pharmaceutical Ingredient (API) and impurity peaks. Adjusting these parameters can therefore introduce a risk and complicate the impurity investigation.
In this case, we opted for the second approach, which offered a key advantage: it allowed us to leverage our in-house LC/MS screening method as the second-dimension LC technique. This setup facilitates a rapid identification as the method is directly coupled to our Nelson Labs’ proprietary screener database.
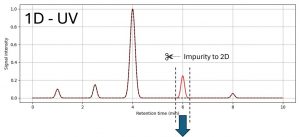
Figure 1: LC/UV spectrum of the routine QC method containing the unknown impurity
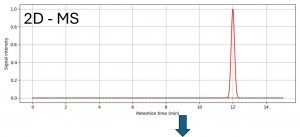
Figure 2: LC/MS chromatogram with a differential MS peak associated to the unknown impurity
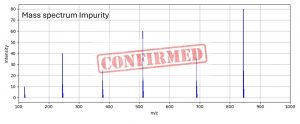
Figure 3: Mass spectrum of the differential MS peak
The result? The unknown impurity was successfully isolated in the second-dimension LC/MS method. Its retention time and mass spectrum produced a successful match with a compound listed in the Nelson Labs’ proprietary Screener Database. This rapid identification enabled to trace the impurity back to its origin—the overpouch, a component of the drug product’s secondary packaging.
This case highlights the importance of advanced analytical techniques and robust databases in modern pharmaceutical QC testing. Even seemingly minor changes in packaging can have significant downstream effects—and being prepared with the right tools makes all the difference.
If you have additional questions about Impurities Identification test services or would like to consult with the experts at Nelson Labs, just send an e-mail to [email protected].



