Blog Post 2 – MIND THE LABEL: rapid impurity identification through in-house intelligence
2 min reading time
Even subtle changes in secondary packaging can introduce unexpected impurities into drug products—posing risks to quality and patient safety. In this first case study of our impurity investigation series, we trace a contaminant back to a label modification and show how the presence of a comprehensive in-house spectral database advances rapid and confident impurity identification.
What’s that peak?
During routine quality control a customer detected an unknown impurity peak in a newly produced batch of drug products. At Nelson Labs, we first successfully reproduced the customers’ Liquid Chromatography (LC) analysis. We observed a differential UV peak versus a blank control sample containing freshly prepared drug product and matrix. Conveniently, this LC method was compatible with a mass spectrometric (MS) analysis. The obtained MS spectra, including fragmentation data, matched with those of a particular compound in our Nelson Labs’ proprietary screener database.
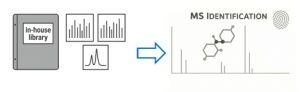
Figure 1: Nelson Labs’ database facilitates MS identification
We further confirmed the identity of the peak of interest by running the sample and its blank with our standard LCMS screening techniques. The extensive stock of certified chemicals maintained by our laboratory was another key factor in this identification. It allowed us to immediately spike the compound of interest to the sample, run it with the customer’s method and unequivocally confirm the identity of the unknown impurity.
Where did it come from?
In this case, the compound of concern was known to originate from paper materials. An extraction of the label of the drug product confirmed this hypothesis. Indeed, the customer discovered that the label appeared to be slightly different from the originally tested label and that this change has slipped through the change control processes.
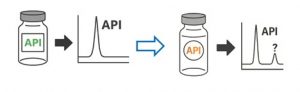
Figure 2: Changes in packaging can cause an unknown impurity peak
What did we learn?
The slightest change in the manufacturing or packaging of pharmaceutical products can have a enormous impact. This particular case study showed that even a change of the labels on secondary packaging can affect the drug product during its shelf life.
Nelson Labs could swiftly assist the customer’s impurity investigation by a combination of state-of-the-art equipment, experienced personnel and its extensive proprietary screener database of relevant mass spectra and stock of relevant extractables and leachables chemicals.
If you have additional questions about Impurities Identification test services or would like to consult with the experts at Nelson Labs, just send an e-mail to [email protected].



