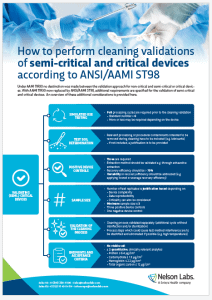
Cleaning validation requirements for reusable medical devices according to AAMI TIR30 did not distinguish between non-critical devices on the one hand and semi-critical or critical devices on the other. Additionally, many validation testing requirements were formulated using generalities rather than specifics. Now that AAMI TIR30 has recently been replaced by ANSI/AAMI ST98 certain aspects of cleaning validations have become more defined, especially for semi-critical and critical devices as summarized in the infographic below.
For example, the standard number of simulated use cycles recommended or the endpoints to be evaluated are now clearly defined. On the other hand, there are no standard recommendations for sample size, which now must be determined by the device manufacturer based on a sample size justification. Our Nelson Labs team of experts can help you perform sample size justifications, leveraging our experience in validation testing and knowledge of how device complexity relates to cleanability.