Blog Post 6 – Database-driven discovery: how NIST23 helped trace a manufacturing impurity
2 min reading time
Impurity identification primarily depends on mass spectrometric analysis and comparison with reference MS spectra. In most cases, impurities are identified by matching spectra against Nelson Lab’s proprietary screening database. This database contains over 8000 spectra representing more than 3000 unique compounds commonly found as extractables or leachables from packaging materials or medical devices. However, as illustrated in this article, identification may also rely on external databases.
In this case study, the customer detected an unexpected impurity in a new batch of their drug product during Quality Control analysis performed with Liquid Chromatography/Ultraviolet (LC/UV). The customer’s LC/UV method was transferred to our own analytical platform, enabling high resolution mass spectrometry detection in series with UV detection. The method transfer was successful as the LC/UV peak of interest was detected with a corresponding MS response.
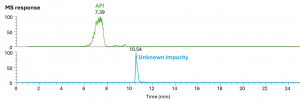
Figure 1: LC/MS response specific for the API & unknown impurity
As a first step in the identification process, the molecular ion was determined to be the most intense ion in the full scan MS spectrum and was confirmed by the presence of two adduct ions. From the accurate mass of the molecular ion, m/z +369.1251, the elemental composition was determined to be C21H21O4P.
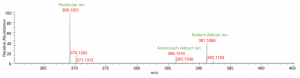
Figure 2: Ion description in MS spectrum of target impurity of interest
The next step in the elucidation process is to acquire MS/MS data, where the molecular ion is isolated and fragmented in the collision cell of the mass spectrometer. The resulting fragment ions make up the MS/MS spectrum that may act as a fingerprint useful for structure elucidation. This fingerprint spectrum can then be searched against reference MS/MS spectra of known organic molecules collected in libraries, aiding in the identification of unknown compounds. One of the best-known mass spectral libraries is the NIST Mass spectral library, where the current NIST23 version includes high resolution MS/MS spectra of 49.950 unique compounds [1].
Upon searching the MS/MS spectrum of the impurity in the NIST database, a good match was obtained with a reference spectrum of a phosphate compound C21H21O4P. The tentative structure proposal was further confirmed by the analysis of an authentic reference standard.
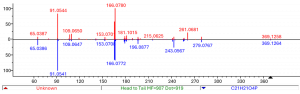
The identified compound is widely known as a plasticizer or flame retardant in plastic materials. Eventually, a root cause analysis pointed towards a worn seal in the manufacturing equipment, releasing the compound into the drug product.
Today, the increasing availability of both licensed & open-source spectral databases are facilitating MS-based structure elucidation of unexpected impurities in drug products. Still, it is the task of the mass spectrometrist to critically review the hits obtained for potentially false positive results. Preferably, the structure proposal from the database should be confirmed with an authentic reference standard. If not available, supporting evidence for the structure proposal could be a fit into the chemical context of the sample investigated, a match with an expected or predicted retention time, an explainable source for the contaminant that emerged, etc.
References:
[1] https://www.nist.gov/srd/nist-standard-reference-database-1a (consulted 20 Jun 2025)
If you have additional questions about Impurities Identification test services or would like to consult with the experts at Nelson Labs, just send an e-mail to [email protected].



