Blog Post 12 – Bridging Analytical Gaps: Identification of GC/MS Impurities from External Labs
3 min reading time
At Nelson Labs, we regularly assist customers with impurity investigations performed either by the drug product manufacturer or another CRO. These cases often present unique challenges, as the analytical methods and reference databases used may differ from those employed at Nelson Labs.
When such inquiries reach our Structure Elucidation team, our workflow typically involves two key steps: method transfer and impurity detection, followed by compound identification. This blog post focuses on transferring external Gas Chromatography Mass Spectrometry (GC/MS) data to our in-house identification platforms.
The first objective is to verify the detection of the impurity using our GC/MS instruments. This is achieved by transferring the method with which the impurity was originally detected. During this process, we focus mainly on method parameters that are critical for the retention time and mass spectrum of the unidentified impurity, such as stationary phase, GC oven program and ionization settings.
In addition to measuring the sample with the transferred method, we also analyze the sample using our default GC/MS screening method to leverage the identification power of our Nelson Lab’s proprietary Screener Database, which is based on retention time-locked data for GC/MS compounds.
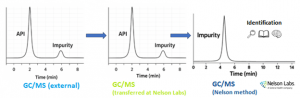
Figure 1: From impurity in external GC/MS data to detection and identification at Nelson Labs
In one case, the impurity was instantly matched with a compound in our Nelson Lab’s GC/MS Screener Database, confirming both retention time and mass spectrum. Remarkably, the compound was not present in commercial libraries like NIST23 or Wiley, thereby reinforcing the advantage of having an extensive internal database.
In another case, no match was found in either in-house or commercial databases. We proceeded with advanced analysis using our Gas Chromatography Quadrupole Time-of-Flight (GC/QTOF) instruments, including Electron Ionization (EI), EI after derivatization, and Chemical Ionization (CI) with methane and isobutane. The combination of these complementary data and expert interpretation led to a tentative identification. The customer then confirmed the identity by analyzing a reference standard using their own method.
These examples highlight the importance of flexible method transfer, comprehensive databases, and multi-technique analysis. At Nelson Labs, our ability to transfer external methods and apply advanced instrumentation ensures accurate impurity characterization—supporting the highest standards in impurity identification.
If you have additional questions about Impurities Identification test services or would like to consult with the experts at Nelson Labs, just send an e-mail to [email protected].



