Medical devices and pharmaceuticals that come into contact with blood, lymphatic or cerebrospinal fluid should be free of harmful levels of endotoxins. Endotoxins are detected using the BET or Limulus amebocyte lysate (LAL) test.
The BET uses the lysate of the amebocytes of the blood of the horseshoe crab. The enzymes in this lysate react in the presence of endotoxins forming a clot or causing a change in color or turbidity which can be observed by either the naked eye (clot) or can be measured quantitatively using a photo spectrometer (color or turbidity).
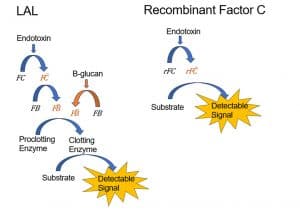
The pathway that leads to these endpoints is a cascade reaction of different enzymes as can be seen in the picture below. The lysate contains the enzymes Factor C, Factor B and the Proclotting enzyme. Endotoxins will cause Factor C to be transferred to Factor C- which will in its turn transfer Factor B to Factor B-. This will activate the clotting enzyme resulting in the end point.
The clotting enzyme can also be activated by factor G that is also present in the lysate. Factor G is activated by Beta-glucans.
Beta glucans are a group of polysaccharides that occur in the cell walls of plants, bacteria, and fungi. The best-known beta-glucan is cellulose. Thus, a product passing through a cellulose filter has the potential to be contaminated with beta-glucans. Since beta glucans activate the clotting enzyme, they will result in the same end point as endotoxins, providing false positive results. Without further thought, the contamination with beta-glucans might remain unseen since contamination cannot be excluded by the quality control checks built into the assay.
During method development, if contamination is suspected, it is important that further investigation is performed. Typically, when testing dilution series of contaminated test items, the results for the dilution series are non-linear. Easily put, if you would find 5 EU/mL in the neat sample, you would expect to find approximately 0.5 EU/mL in the 10x diluted sample and 0.05 EU/mL in the 100x diluted sample. The concentration responses for beta-glucans often deviate from this linearity. Positive product controls greater than 200% may also be an indicator of beta-glucans.
Fortunately, there are means of overcoming this contamination or interference.
The most common way to overcome interference due to beta-glucans is the use of a Beta-Glucan Blocker. Adding this blocker to the reaction medium will prevent the Factor G pathway from being activated.
Another possibility is the use of the recombinant Factor C assay instead of the BET.
Recombinant Factor C does not contain the Factor G enzyme and is therefore not affected by beta-glucans.