
In January 2023 the ASTM F23 Biological Committee balloted to revise the Sub-Micron Filtration Efficiency Test Method used in ASTM F2100. This change was reflected in the new revision published on 01 March 2023 and updated the Particle Filtration Efficiency (PFE) test from ASTM F2299, Standard Test Method for Determining the Initial Efficiency of Materials used in Medical Face Masks to Penetration by Particulates Using Latex Spheres, to ASTM F3502, Standard Specification for Barrier Face Coverings.
There are several differences across the two test methods, some of which are demonstrated in the table below:
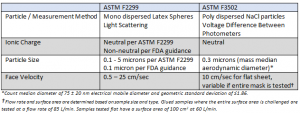
The performance requirements were changed to align with the NaCl method, as this method is more rigorous than the ASTM F2299 test. The updates to these performance requirements are reflected in the table below.
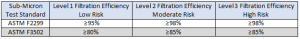
Refer to this article https://www.nature.com/articles/s41597-022-01860-y for more data supporting the specification update and insights into the real-world performance of medical face masks, including the different ways of measuring sub-micron filtration efficiency, which can cause differences in results.
Why the Test Methods Changed
There are a couple of important reasons these test methods were changed. First, this allows the industry to more accurately compare the sub-micron filtration efficiency of medical face masks to other product types, such as barrier face coverings and respirators, as the methods are similar.
Second, F3502 (NaCl method) is more controlled. Parameters are set and are, therefore, less variable between labs when compared to ASTM F2299 (latex particle method).
Parameters that are important to control:
- Face Velocity: Face velocity is the speed at which the air is flowing through the material and is calculated by dividing the flow rate by the test area during testing. Results are impacted by face velocity; different face velocities will either increase or decrease filtration efficiency results. A face velocity of 5 cm/s will achieve better results than a face velocity of 10 cm/s, as a higher face velocity is more severe.
- Charge neutralization: Neutral vs. nonneutral will also change filtration efficiency results. Charged particles (nonneutral) are easier to capture due to electrostatic attraction.
- Particle size: Both F2299 and F3502 NaCl method use sub-micron particles, which are harder to capture than larger particles. Using different particle sizes can either increase or decrease filtration-efficiency results.
What Face Mask Manufacturers Need to Know
Effective as of July 2025, the FDA is only accepting sub-micron results tested according to ASTM F3502. However, Nelson Labs will continue to offer ASTM F2299 for the foreseeable future to support clients as needed.
If you have questions on what testing should be performed for your submission, it is recommended you contact your FDA representative.
Our Nelson Labs experts are also happy to assist you with this process. If you have any questions, feel free to contact [email protected].



