Sterility assurance is of utmost importance for medical devices that come into contact with body cavities. However, bioburden and sterility are linked to a third concern—pyrogens—which may not be adequately addressed solely by ensuring device sterility.
Pyrogens and Endotoxins: Understanding the Terminology
Pyrogens are substances that induce fever when they are present in the blood stream or cerebrospinal fluid (found in the brain and central nervous system). The development of the first parenteral solutions, like glucose for injection, took place in the early 1900s. An associated issue known as injection fever emerged during that time. Years later the link was demonstrated between bacterial contamination and injection fever.
Pyrogens are bacterial in nature, with the most common type of pyrogens being endotoxins derived from Gram-negative bacteria. Therefore, all endotoxins are considered pyrogens, but not all pyrogens are necessarily endotoxins.
To gain a better understanding of pyrogens and endotoxins, it is important to note that bacteria can be classified into two groups based on their cell wall: Gram-positive and Gram-negative strains. Gram-positive bacteria get their name from the fact that they retain a purple color when subjected to the Gram stain test, while Gram-negative bacteria retain a pink color (see Figure 1).
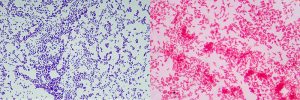
The fact that these two types of bacteria show different colors in the Gram stain test stems from the fact that the cell walls of these two types differ significantly. Gram-positive bacteria have a single cell wall constructed of a double-layered phospholipid membrane, covered mainly by lipoteichoic acid and peptidoglycan. Gram-negative bacteria have a double cell wall consisting of a double-layered phospholipid membrane on the inside and a lipopolysaccharide membrane on the outside (see Figure 2).
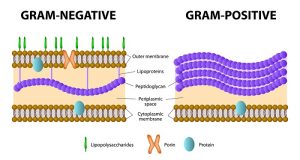
Lipopolysaccharides (LPS) are the most resistant and most reactive (up to one thousand times more reactive in rabbits) of all known pyrogens and are referred to as endotoxins and, as a result, are the most investigated during quality-control testing of healthcare products.
The Relationship Between Pyrogens, Bioburden, and Sterility
Given that pyrogens are derived from bacteria, pyrogen contamination is always linked to bioburden contamination. When bacteria are inactivated, the pyrogens remain fully active. Common sterilization methods, such as ethylene oxide (EO), gamma radiation, and moist heat, are not effective in destroying significant levels of endotoxins. As a result, sterile samples do not guarantee the absence of pyrogens. Thus, sterile samples do not necessarily mean that samples are microbiologically safe. It is important to understand that pyrogens should always be investigated in addition to sterility testing for products that come into contact with circulating blood and cerebrospinal fluid.





