Blog Post 5 – A spectrum of clues: identifying residual solvent impurities using diverse analytical tools
2 min reading time
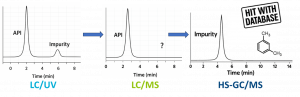
Figure 1: Overview of different techniques used to identify the unknown
In pharmaceutical manufacturing, controlling impurities is essential to ensure drug safety and efficacy. However, some impurities originate from unexpected sources, making their identification particularly challenging. One such group of impurities consists of residual solvents, which can be introduced at various stages of production or packaging. These solvents may stem from seemingly unrelated materials such as ink used in labeling, cleaning agents utilized in facility maintenance, or even paint from production equipment.
In this case study, the client detected an impurity using Liquid Chromatography/Ultraviolet (LC/UV) analysis during their routine control. In the attempt to identify the impurity, the sponsor’s LC/UV method was transferred to our high-end LC/MS platform, enabling high-resolution Mass Spectrometry (MS) based characterization.
In our first attempt, we linked the UV-detected impurity to the sponsor’s chromatogram, confirming that the LC/UV results matched and that the method transfer was successful. With this correlation established, the next step was to find any corresponding mass spectrometry signals. Despite extensive efforts—testing multiple ionization techniques (including Electrospray Ionization (ESI) and Atmospheric Pressure Chemical Ionization (APCI)), optimizing sample concentration, and increasing injection volumes—no MS signal was detected at the retention time of the UV peak L. A reconsideration of the analytical approach was necessary, prompting us to explore alternative techniques for impurity identification.
To this end, we switched from liquid to Gas Chromatographic techniques (GC/MS and headspace GC/MS), which are well-suited for detecting more volatile compounds that may be missed by LC/MS detection. While standard GC/MS screening yielded no significant findings, headspace GC/MS analysis identified xylene as a differential compound. The impurity was recognized using our Nelson Labs’ proprietary screening database, which leverages a broad reference library and deep expertise in packaging-related impurities. The identification was further confirmed with LC/UV analysis utilizing a xylene reference standard.
Xylene exhibits high sensitivity to UV detection but remains largely undetectable via LC/MS due to its low molecular weight, volatility, and non-polar nature. This case demonstrates the importance of integrating multiple analytical techniques to characterize pharmaceutical impurities. By using a diversified approach, manufacturers can achieve a more thorough impurity analysis, ultimately enhancing drug product safety and quality.
At Nelson Labs, we specialize in identifying even the most unexpected impurities through advanced analytical techniques, a broad screening database, and extensive knowledge of packaging-related contaminants. These capabilities enable us to support clients in maintaining the highest standards of pharmaceutical quality control.
If you have additional questions about Impurities Identification test services or would like to consult with the experts at Nelson Labs, just send an e-mail to [email protected].



