
During their Extractable & Leachable Qualification work, many Pharma and Biopharma companies qualifying their Small Volume Parenteral Container Closure systems (vial, syringe, or cartridge) may have come across certain compounds that they now recognize as rubber oligomers. While the exact structure of these compounds remained unknown for a long time, a lot of progress has been made during the last decade – or so – toward the structural elucidation of these compounds. This was important as some of these oligomers – based upon mass-spectral information — appeared to be either brominated (oligomers from bromobutyl rubbers) or chlorinated (oligomers from chlorobutyl rubbers). The fact that these compounds are either brominated or chlorinated raises the concern about this class of oligomers possibly being present as a leachable from a toxicological perspective.
This concern resulted in the full structural elucidation of these oligomers, which allowed toxicologists to link these obtained structures to their associated toxicological information. Because there was little known about these compounds, it was evident that there was no literature or experimental toxicological data available on them, so the only alternative to assess their toxicity was to perform a (Q)SAR assessment on their chemical structure (i.e. Derek, Leadscope and MultiCase).
The outcome of the (Q)SAR assessment revealed that this class of compounds should be considered as alkylating agents, which then leads to the possibility that they may be carcinogenic. While it is true – and observed many times (this will be a subject of one of the future blogs) — that these compounds are highly reactive (cases show their reactivity with drug product constituents), the assumption of these compounds being carcinogenic has not been evaluated in the public domain.
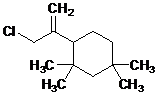
In 2012, we took the initiative to isolate these compounds from rubber to allow us to do further work on the characterization of these compounds, and to use them as analytical reference standards in the supporting Extractable & Leachable studies we perform for our customers. However, the amounts we could obtain via this isolation procedure was just too low to perform an AMES test. The good news is that earlier this year (2019) we finally had sufficient amounts of the C13H23Br and C13H23Cl oligomers to perform the AMES tests.
With this blog, I am extremely proud to announce that Nelson Labs has completed the GLP – AMES testing and we have finalized the study reports. These new findings will allow pharmaceutical companies to better identify and understand the mutagenic/carcinogenic potential of their container closure systems and include this information into a final safety assessment.
If anyone is interested in the AMES reports of the C13H23Br and C13H23Cl, please feel free to contact Kevin Breesch ([email protected]). He will be happy to see how we can help you with this subject.
Have great day! Piet


