Introduction
The design, validation and regulatory review of orthopaedic trays and instruments are a sophisticated challenge. Many U.S. companies successfully compete in this industry in part because of the helpful guidance available from FDA, United States Pharmacoepeia (USP) and ISO. Contract laboratories play an important role in this process. This case study, “Sterilization by Moist Sterilization at 132°C of Reusable Device and Tray System,” systematically investigates the role of steam, heat and load configuration in the validation of moist heat sterilization and drying.
Method
The orthopaedic instrument system comprised six different reusable instruments. The first step was to determine whether or not the instrument system could be successfully steam sterilized under the worst case conditions according to ST79.1 The validation was performed in a validated hospital sized gravity and pre-vacuum autoclave,, with FDA-cleared approved 510(k) sterilizer.2 Heat penetration studies were preformed on each reusable instrument by placing a National Institute of Standards and Technology thermocouple inside each instrument. Pre- and post-thremocouple calibration was performed. The instruments were individually wrapped with a 24 x 24 inch Convertors Bio-Shield Sterilization wrap, and pulsed pre-vacuum and gravity-displacement sterilization cycles were performed. The instruments were placed in the steam sterilizer’s previously-determined cold spot.3,4 To assess sterilization effectiveness, half-cycle sterilization at 132°C for two minutes and 7.5 minutes were performed for pulsed pre-vacuum and gravity-displacement sterilization cycles, receptively as delineated in Table 1.
Table 1: Steam Sterilization Parameters1
| Pulsed Pre-vacuum | Gravity-Displacement | |
| Preconditioning pulses | 4 | None |
| Exposure Temperature set point | 123°C (270°F) | 132°C (270°F) |
| Exposure time (half-cycle) | 2 minutes | 7.5 minutes |
| Dry time | 0 minutes | 0 minutes |
| Exposure time (full-cycle) | 4 minutes | 15 minutes |
Biological challenges were preformed to verify half-cycle sterilization cycles1 effectiveness under both maximum and minimum load configurations. (Refer to Exhibits 1 and 2) Geobacillus stearothemophilus at 2×105 spores per indicator, having a D121-value of 2.2 minutes and a Z value of 9C, were used.4 One biological indicator was inserted into multiple locations of each instrument. These locations were non-surface portions of the instrument, so as to achieve maximum retention of and barrier effects to the biological indicator. The maximum load comprised three trays and three baskets each fully loaded with surgical instruments. The six test instruments were placed in the immediate proximity of the cold spot at the back right-hand top shelf of the chamber. The minimum load configuration was six reusable instruments, individually wrapped and placed on the right side of the top shelf at the back of the chamber. After the cycle, the biological indicators were aseptically removed and cultured at 55-60°C for even days in USP soybean casein digest broth.5
Full cycle sterilization runs were performed to assess whether the ST79 requirements of dryness were met, as delineated in Table 2.
Table 2: Drying Parameters
| Sterilization Process | Exposure temperature setting | Preconditioning pulses | Exposure time | Drying time | Comments |
| Pulsed Pre-vacuum | 132°C | 4 | 4 minutes | 30 minutes | 15-minute cooling off with autoclave door slightly open |
| Gravity Displacement | 132°C | None | 15 minutes | 15 minutes | 15-minute cooling off with autoclave door slightly open and 15 minutes at room temperature |
Results
The thermocouples used for thermal mapping conformed to the pre- and post-calibration requirements of ± 0.5°C.6 The cold spot inside the reusable instruments at the sterilization chamber’s cold spot was determined by thermocouple mapping. As indicated in Table 3, TC06 was found to to be the cold spot for the pulsed pre-vauum sterilization process, and TC06 werefound to be the cold spots for the gravity displacement sterilization process.7
Table 3: Summary of the Cold Spot for the Reusable Instruments Under Maximum Load
| Pulsed Pre-vacuum cycles
(132°C, 4 minutes) |
Gravity-Displacement cycles
(132°C, 15 minutes) |
||
| Avg. temp. (°C) | Cold TC | Avg. temp. (°C) | Cold TC |
| 132.3 | TC06 | 132.6 | TC05, TC06 |
| The exposure temperature setting of 132°C was achieved in all locations of the chamber. | |||
For five of the six instruments in all of the half-cycle sterilization processes, all of the Biological Indicators (BIs) were sterilized. One instrument (#4) could not be sterilized by the pulsed pre-vacuum sterilization process. The positive BI control and negative media control were positive and negative, respectively, for growth as expected. (See Table 4.) The failed instrument was retested with more space available in the are of the BI by not having the handle completely closed, as illustrated in Exhibit 3; 0/2 BI were positive upon retest, as shown in Table 5.
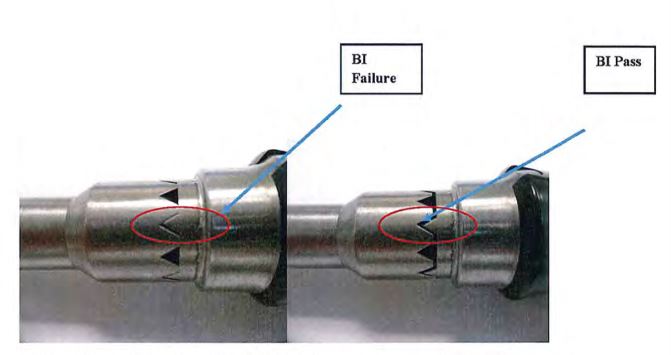
Exhibit 3: Reconfiguration (right side) of the instrument to permit greater steam access
The label claim population of the BIs conformed to the USP specifications of not less than 0.30 log10 and not more than 0.48 log10 of Label Claim. The log number of spores per carrier at 48 hours in equal to or greater to the log number after 24 hours.5
All Positive BIs were found by gram stain as Gram Positive Bacillus, and further identified to the expected species level using Vitek Compact 2.
Moisture was not observed on wrap, or Reusable Instruments wrapped individually in double 24″x24″ Convertors Bio-Shield Sterilization Wrap for three full-cycles for pulsed pre-vacuum and gravity displacement sterilization processes, as delineated in Tables 6 and 7.
Exhibit 1: Maximum Load Chamber Configuration
Note: Six instruments (individually wrapped) were placd on the right side of the top shelf (the cold spot of the chamber) and filled in with three additional fully-loaded instrument trays and three surgical washing baskets with instruments loaded inside the chamber.
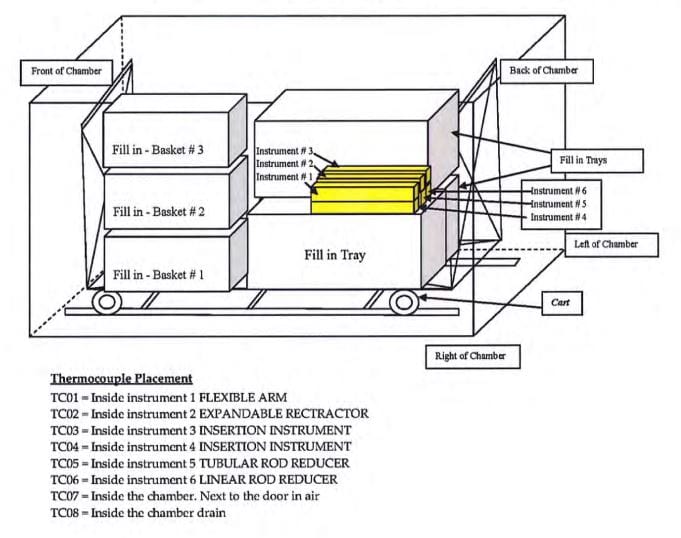
Exhibit 2: Minimum Load Chamber Configuration
Note: Six instruments (individually wrapped) were placed on the right side of the top shelf (the cold spot of the chamber).
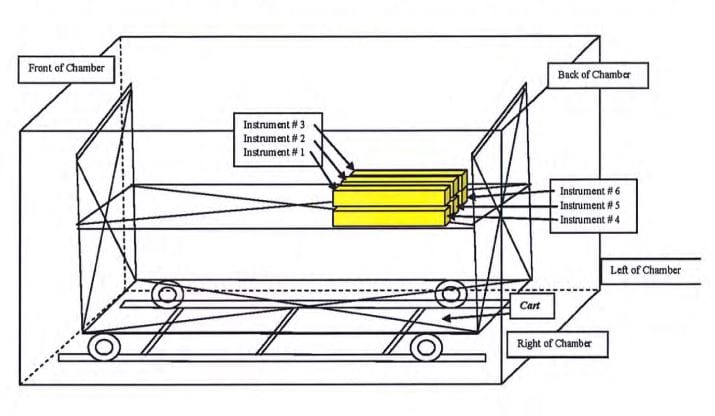
Table 4: Pulsed Pre-Vacuum Number Positive/Number Tested Results (2 minute Half-Cycle)
| Instrument 1 | Instrument 2 | Instrument 2 | Instrument 4 | Instrument 5 | Instrument 6 | ||
| Maximum Load 1 |
0/6 |
0/5 | 0/3 | 0/2 | 2/6 | 0/2 |
0/3 |
| Maximum Load 2 |
0/6 |
0/5 | 0/3 | 1/2 | 0/2 |
0/3 |
|
| Maximum Load 3 |
0/6 |
0/5 | 0/3 | 1/2 | 0/2 |
0/3 |
|
| Minimum Load 1 |
0/6 |
0/5 | 0/3 | 1/2 | 3/6 | 0/2 |
0/3 |
| Minimum Load 2 |
0/6 |
0/5 | 0/3 | 1/2 | 0/2 |
0/3 |
|
| Minimum Load 3 |
0/6 |
0/5 | 0/3 | 1/2 | 0/2 |
0/3 |
|
Positive and Negative controls produced results as expected. Instrument #4 alone displayed resistivity, 5/12 positive. All BIs were killed in the gravity displacement 7.5 minute half-cycle (data not shown).
Table 5: Pulsed Pre-Vacuum Number Positive/Number Tested Results Reconfigured Instrument 4 (2 minute Half-Cycle)
Reconfigured Instrument 4 (2 minute Half-Cycle)
| Load Configuration | Sterilization Process | Number BIs Positive | |
| Maximum Load 1 | Pulsed Pre-vacuum |
0/2 |
0/6 |
| Maximum Load 2 | Pulsed Pre-vacuum |
0/2 |
|
| Maximum Load 3 | Pulsed Pre-vacuum |
0/2 |
|
| Minimum Load 1 | Pulsed Pre-vacuum |
0/2 |
0/6 |
| Minimum Load 2 | Pulsed Pre-vacuum |
0/2 |
|
| Minimum Load 3 | Pulsed Pre-vacuum |
0/2 |
|
Positive and Negative controls produced results as expected. Reversal of BI resistivity in instrument #4 was obtained, 0/12 positive.
Table 6: Dry Time Parameters and Results for Instruments #1-#6 (Pulsed Pre-vacuum); Average of six instruments in three unique cycles
| Full Cycle Parameters = Pulsed Pre-vacuum; 132°C(270°F) – Exposure Temperature; 4 pre-conditioning pulses; 4 minutes Exposure Time, 30 minutes Dry Time, with additional cooling off period of 15 minutes to the autoclave with the door slightly opened and an additional holding time of = 15 minutes at room temperature. | |||||
|
Weighted Wrap Before Sterilization |
Weighted Wrap After Sterilization | % Weight Change | Weight Wrapped Implants Before Sterilization Cycle | Weight Wrapped Implants After Sterilization Cycle |
% Weight Change |
|
37 g |
36 g | 2.07 | 648 g | 646 g |
0.37 |
Table 7: Dry Time Parameters and Results for Instruments #1-#6 (Gravity Displacement); Average of six instruments in three unique cycles
| Full Cycle Parameters = Pulsed Pre-vacuum; 132°C(270°F) – Exposure Temperature; 4 pre-conditioning pulses; 4 minutes Exposure Time, 30 minutes Dry Time, with additional cooling off period of 15 minutes to the autoclave with the door slightly opened and an additional holding time of = 15 minutes at room temperature. | |||||
|
Weighted Wrap Before Sterilization |
Weighted Wrap After Sterilization | % Weight Change | Weight Wrapped Implants Before Sterilization Cycle | Weight Wrapped Implants After Sterilization Cycle | % Weight Change |
| 37 g | 36 g | 2.65 | 648 g | 646 g |
0.36 |
Discussion
Orthopaedic instruments are complex, with multiple locations in which tissue-related organic material may become embedded, such as in a groove or cannulated are. Sterilization of the outer surface of the instrument is not adequate to protect the patient from exposure to infectious material. In the work described here, worst case conditions were employed in the moist heat sterilization validation scheme. Specifically, (1) relatively resistant bacterial spores were inserted into the instrument at locations judged to create the greatest barrier to the penetration by steam, (2) half the anticipated sterilization exposure time was used to determine spore death and (3) maximum and minimum loads were separately sterilized.
It is not well-know whether moist heat sterilization is more readily achieved under maximum or minimum load configurations. The maximum load was a fully-loaded chamber containing three trays and three fill-in baskets each containing the six instrument. The BIs were located in the tray positioned in the chamber’s coldest spot. For the minimum load, the chamber contained only six instruments located in the immediate proximity of the chamber’s coldest spot and there were not trays or baskets. More BI failure was common to a single instrument, #4. This instrument is the type that can be actuated to an open and closed position. Initially, it was tested in the closed position and failed. When it was tested in the open position, it passed.
Thermal mapping of this location demonstrated that the exposure temperature of 132°C was slightly exceeded for this and all of the other five instruments. Interestingly, the overall time of the half-cycle was much longer for the maximum versus minimum load configurations, 29.7 minutes and 19.9 minutes, respectively. Accordingly the failure was due to the fact that the location of the BI was such that insufficient steam could access it. The failure was only apparent from the biological testing, as F0 heat lethality alone is not predictive of the results obtained. Therefore, it is apparent from this case study that biological testing is an essential component of a validation plan. The failure was easily overcome by creating more steam access channels, as demonstrated in Exhibit 3. An exaggerated minimum load half-cycle comprising the failed instrument and one other instrument positioned at the chamber’s coldest spot, rather than six instruments, was also successfully sterilized. In this study, the minimum pulsed pre-vacuum load was a more stringent sterilization condition; however, we have observed the opposite in other studies.8 Accordingly, this result is not a universal finding, but is a distinct possibility revealing that a physical determination may not necessarily supersede a biological determination in validation studies as reported here.
No failure was observed in any of the Gravity-displacement sterilization processes. (Refer to Table 4) Comparing the full-cycles of the Pulsed pre-vacuum to the Gravity-displacement, the calculated F0 values were five minutes and 18 minutes 40 seconds, respectively consistent with the results obtained. It is noted that the 7.5-minute half-cycle was substantially longer than the two-minute pulsed pre-vacuum exposure.
Drying was achieved for all full-cycle sterilization processes. (Refer to Tables 6 and 7) There was no evidence of moisture on the sterilization wraps or reusable instrument following the exposure to the full-cycle sterilization processes. In addition, with respect to moisture retention following sterilization, (1) the wrap weight did not increase by more than 3% relative to its weight before sterilization (ANSI/AAMI ST70 and (AAMI TIR 12) and 4 the wrapped tray did not increase by more than 0.2% relative to its weight before sterilization (EN-868-8).9
REFERENCES
- ANSI/AAMI ST79:2010 and A1:2010 – Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
- ANSI/AAMI ST8-2008 Hospital Steam Sterilizers.
- ANSI/AAMI ST 46-2002. Good Hospital Practice: Steam Sterilization and Sterility Assurance.
- AAMI TIR 12-2010 Designing, testing and labeling reusable medical devices for reprocessing health care facilities: A guide for medical device manufacture.
- Current United States Pharmacopeia Chapter <55> Biological Indicators – Resistance Performance Tests.
- ANSI/AAMI/ISO 18472:2006(R) 2010 – Sterilization of health care products – Biological and chemical indicators – test equipment.
- ANSI/AAMI ST77:2006(R) 2010. Containment devices for reusable medical device sterilization.
- Gibraltar Laboratories, Inc. #3193605/1.409
- EN 868-8:2009. Packaging materials for terminally sterilized medical devices Part 8: Re-usable sterilization containers for steam sterilizers conforming to EN 285 – Requirements and test methods. European Committee for Standardization, Brussels, Belgium.
Daniel Prince, Ph. D., is a respected specialist in the fields of microbiology and molecular biology, who received his doctorate from Rutgers University/University of Medicine and Dentistry of New Jersey and as president of Gibraltar Research Laboratories., has led the company’s growth and expansion since 1987.
Josef Mastej, who serves as Vice President of Operations at Gibraltar Research Laboratories, Inc., has been with the company for 18 years and is a well-known professional in the field of steam sterilization, cleaning, microbiology and sterility.
Diana Easton, Ph. D., has twenty years of experience in the medical device industry and holds her Ph. D. in Systems Engineering. She is currently the Vice President of Quality and Regulatory Affairs at Orthofix.
Isabel Hoverman has more than twenty years of experience in sterilization processes and holds her Bachelor of Science in Microbiology. She is currently a Quality Engineer at Orthofix.
Raja Chatterjee holds a Master’s degree in Mechanical Engineering and currently holds the position of Director Product Quality at Orthofix.


