In the previous two posts, the biocompatibility of 3D printed devices was discussed with consideration for the possible compounds added to the raw materials for workability and the polymer precursors and byproducts associated with photopolymerized structures. In both of these cases, the discussion focused on the materials designed to be part of the final structure. Here, the introduction of compounds from the sacrificial support material, post-printing rinsing, and finishing processes are discussed; all of which are secondary to the device material itself.
3D printing offers facile creation of complicated devices by depositing the structure additively along with a selectively removable support material. The support material allows the printing of overhanging parts by providing a structural platform for the device material, and can act as a thin layer between parts that are printed very close to each other but should be prevented from fusing (think, for example, of printing a device with movable gears). Without sacrificial support materials, 3D printed designs would be severely limited. Because the support material is not intended to be part of the finished device, it may be overlooked as a possible source of biocompatibility issues.
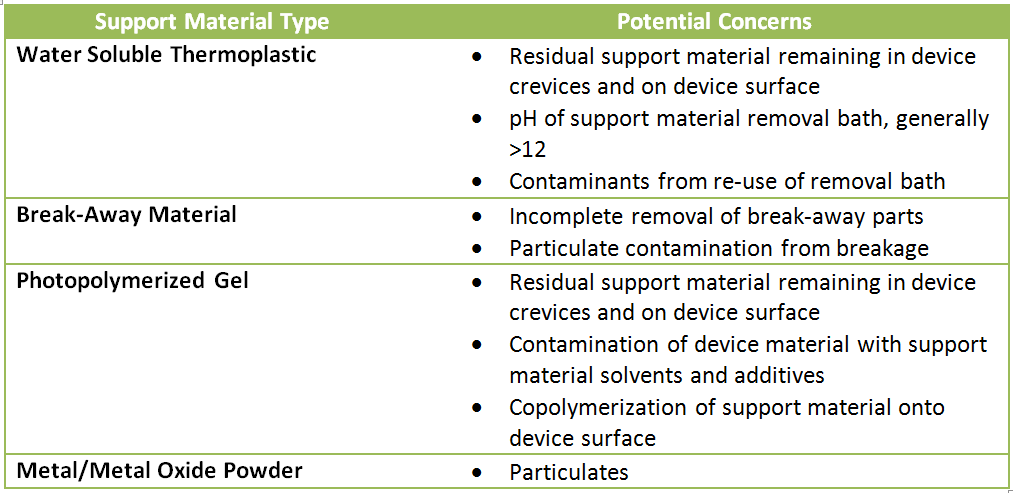
After printing, the sacrificial support material (which is generally dissolvable in a water based cleaning solution) must be removed. The compatibility of the support material depends on the printing technology used. Methods that deposit thin lines of thermoplastic use a special water-soluble polymer or break-away material, while photopolymerization methods may use a loosely polymerized gel or unexposed photopolymer as support materials. Laser sintering methods (often used to produce metal and metal oxide parts) use un-sintered precursor powder.
Each support material and removal method raises potential concerns from a biocompatibility perspective.
Following removal of support materials, 3D printed devices may undergo subsequent finishing processes. Extruded thermoplastics may be smoothed through exposure to heated solvent vapors such as acetone or methylene chloride. The combination of heat and natural affinity of the solvent for the material creates ideal conditions for adsorption into the material surface. Metal parts may undergo passivation processes that introduce surface contaminants. Desorption of volatiles from 3D printed material will be discussed next week.


